Which of the Following Correctly Describes Chemical Equilibrium
The reaction is now irreversible. Which of the following statements is true for a system at chemical equilibrium.
The rate of the forward reaction is equal to the rate of the reverse reaction.

. B there is no change in the concentration of reactants or products. The equilibrium constant K P for the reaction N 2 g 3H 2 g 2NH 3 g is 16 10-4 atm-2 at 400 o C. C two reactions are occurring simultaneously.
1 and 2 D. A the forward and reverse reactions are occurring at the same rate. Both forward and reverse reactions have halted.
The forward and reverse reactions occur at equal rates. The ratio productsreactants remains constant although forward and reverse reactions are still occurring. There are many examples of chemical equilibrium all around you.
2 More liquid water molecules will change to water vapor until a new equilibrium is reached. Which one of the following statements correctly describes chemical equilibrium. Reactions stop only when all reactants have been.
Which of the following options correctly describe the state of chemical equilibrium. 4 The additional bromine ions cause the equilibrium to shift to the reactants. What will be the equilibrium constant of the Chemical equilibrium at 500 o C if the heat of the reaction at this temperature range is.
For a system at. 3 by adding water h2O. Select all that apply.
1 and 3 C. 3 The concentrations of reactants and products remain constant. Which of the following statements correctly describes any chemical reaction that has reached equilibrium.
Which statement does not correctly describe a chemical system at equilibrium. Get Answers Chief of LearnyVerse. 1 2 and 3 B.
Which of the following options correctly describe the ionization of an acid in aqueous solution. A reaction is in chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. Select all that apply.
Which of the following correctly describes the equilibrium constant for the gas phase reaction between J_2 and O_2 form gaseous H_2O. Which of the following statements correctly describes a chemical system that has reached equilibrium. In the bottle there is carbon dioxide CO2 dissolved in the liquid.
Chemical equilibrium is the state of a system in which the rate of the forward reaction is equal to the rate of the reverse reaction. Which of the following are strong electrolytes. One example is a bottle of fizzy cooldrink.
Forward and reverse reactions have stopped so that the concentration of the reactants equals the concentration of the products. The reaction is now irreversible. 231k points 91 992 3029.
Problems on Chemical Equilibrium. Which of the following statements correctly describes any chemical reaction that has reached equilibrium. Both forward and reverse reactions have halted.
2 No further macroscopic changes in the system are observed. The concentrations of products and reactants are equal. Which of the following options correctly describe the equilibrium constant K.
The rates of the forward and reverse reactions are equal. The concentration of products and reactants are equal. The rates of the forward and reverse reactions are equal.
Which of the following statements correctly describes any chemical reaction that has reached equilibriumA The concentration of products equals the concentration of reactantsB The rate of the forward reaction equals the rate of the reverse reactionC Both forward and reverse reactions have haltedD The reaction is now irreversibleE No reactants. 1 The rate of the forward reaction equals the rate of the reverse reaction. Answers 1 Ulises Powell 25 July 0517.
Which of the following statements correctly describe a chemical system at equilibrium. 1 The forward and reverse rates of reaction are equal. D no forward or reverse reaction is occurring.
The general formula HX is often used to represent an acid. Select all that apply. Concentrations of products are higher than the concentrations of the reactants.
-K kfwdk krev for a system at equilibrium. Ammonia is formed in the Haber process according to the following balanced equation N 2 3H 2 2NH 3 The table shows the percentages of ammonia present at equilibrium under different conditions of temperature T and pressure P when hydrogen and nitrogen gases were mixed in a.

8 2 Chemical Equilibrium Chemistry Libretexts

Solved Ooo 15 Of 50 Which Of The Following Correctly Chegg Com
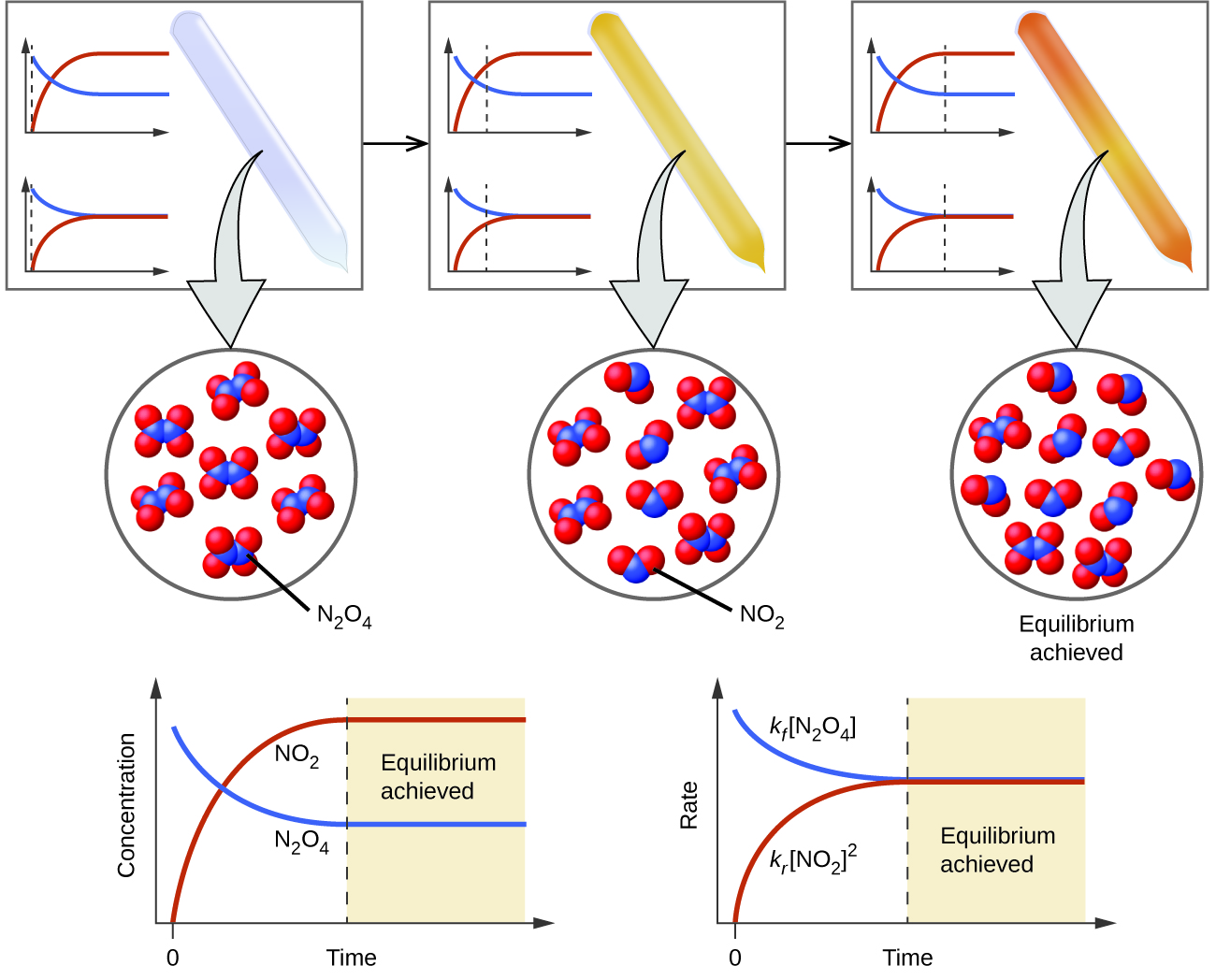
13 1 Chemical Equilibria Chemistry

Chemical Equilibrium Types Problems Factors Affecting Examples
No comments for "Which of the Following Correctly Describes Chemical Equilibrium"
Post a Comment